Backround:
Thromboembolism occurs in about 10% of patients with multiple myeloma (MM). The incidence of VTE is higher in newly diagnosed MM patients as compared to those with relapsed or refractory disease and it is higher during the first 3 to 6 months after the initial diagnosis and treatment initiation. Some of the treatments administered to patients with MM are independent risk factors for VTE. Immunomodulatory agents (IMiDs) among anti-myeloma treatments stand out as having a considerable prothrombotic effect. Recognizing the significant risk associated with the use of immunomodulatory agents (the International Myeloma Working Group (IMWG) 2014 statement, and the European Myeloma Network Guidelines in 2015 both included guidance on the prevention of VTE in MM patients who receive IMiDs. The prospective, longitudinal observational study ROADMAP-MM CAT was designed to explore alternative strategies for the development of risk stratification tools in patients with multiple myeloma. To this target we evaluated the baseline profile of hypercoagulability in multiple myeloma patients with various disease status. Blood borne hypercoagulabity partly consisted of enhanced thrombin generation, a common phenomenon in patients with malignancies . It remains to be seen whether this phenomenon appears for multiple myeloma patients during the physical course of the disease.
Aim: We conducted a study to explore the relationship between stages of MM and alterations of various thrombosis-related biomarkers in patients with MM and their relationship with MM therapy.
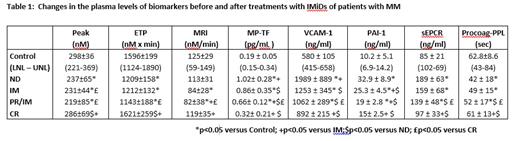
Materials and Methods Patients with MM (n=162) were recruited and stratified to the following groups: 59 newly diagnosed treatment-naïve patients (ND), 49 patients receiving IMiDs (IM), 52 in complete remission (CR) and 12 patients in partial remission on IMiDs (PR/IM). Patients on anticoagulant treatment were excluded from the study. The control group (CG) consisted of 30 healthy age and sex-matched individuals. Samples of platelet-poor plasma (PPP) were assessed for thrombin generation (TG) with the TF 5pM PPP-Reagent® on Calibrated Automated Thrombogram (Diagnostica Stago, France). Plasminogen activator inhibitor-1 (PAI-1), soluble endothelial protein C receptor (sEPCR), and soluble vascular cell adhesion molecule-1 (sVCAM-1), Procoag-PPL were measured using antibody-based ELISA kits (Invitrogen International Inc., CA, USA, and Diagnostica Stago, France ). TF expression MPC-derived microparticles (MPC-dMPs) were detected using a Zymuphen MP-TF activity kit (Hyphen BioMed, Neuville sur Oise, France). The upper and lower normal limits (LNL and UNL) were calculated by the mean±2 SD.
Results A total of 162 patients were enrolled (age 66.0±12.0 yrs; 53% male). Distribution of disease stage was as follows: 32% ISS I, 23% ISS II, 45% ISS III. Bone disease was present in 71% of patients and 19% of patients had high risk cytogenetic lesions. Patients with ongoing MM (ND, IM, PR/IM) had significantly lower Peak, ETP and MRI as compared to the CG. In contrast, patients in CR had Peak, ETP, MRI values similar to the CG. Patients with PR had lower ETP and MRI values as compared to the CR group. In ND 9% had TG >UNL and 22% had TG<LNL. In IM 10% had TG>UNL and 67% had TG<LNL. None in PR/IM had TG>UNL and 35% had TG<LNL. In CR, 23% had TG>UNL and 12% had TG<LNL. MP-TF levels and PPL were higher in untreated MM (CR) patients compared to control. MP-TF and PPL levels decreased significantly after chemotherapy in PR/IM but MP-TF remained high at CR. The plasma concentrations, PAI-1, sEPCR, and sVCAM-1 were higher in MM patients than controls. , Treatment with IMDs reduced all biomarker levels gradually from PR/IM to CR (Table 1).
Conclusion Patients with active MM showed attenuated TG which was enhanced in the presence of IMiDs treatment. Complete remission was associated with normalization of TG. The plasma concentrations, TF-MP , PAI-1, sEPCR, and sVCAM-1 were increased in MM patients suggesting vascular aggression which is followed by release of these markers that could contribute to the procoagulant environment. Attention should be paid to therapy-related thrombosis indeed the effect the suggested role of IMiDs in hypercoagulability does not seem to be confirmed and requires further studies .
Disclosures
Kastritis:Janssen: Honoraria, Research Funding; Sanofi: Honoraria; Pfizer: Honoraria, Research Funding; GSK: Honoraria, Research Funding. Terpos:Sanofi: Honoraria, Other: Travel expenses, Research Funding; Amgen: Honoraria, Other: Travel Expenses, Research Funding; BMS: Honoraria; ASTRA/Zeneca: Honoraria, Other: Travel Expenses; EUSA Pharma: Honoraria, Other: Travel expenses; GSK: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Menarini/Stemline: Honoraria; Pfizer: Honoraria; Takeda: Honoraria, Other: Travel expenses, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal